Chirality, enantiomers, epimers
The word chirality, comes from the Greek χειρ (kheir) meaning “hand”.
It refers to molecules in which the atoms, or groups of atoms, that make up them, can be arranged in space in an asymmetric manner. There are thus pairs of molecules that use the same atoms but are specularly arranged in space. Just like the two hands that can not be superimposed identically to each other but they can “look themselves in the mirror“. Such molecules are referred to as enantiomers.
Enantiomericity is a particular case in which the configurations are two and are mirrored one another. Making it more general, there may be molecules in which the atoms are linked in the same way, but with different orientations and not always these arrangements are such as to produce specular copies.
These molecules are called stereoisomers and non-overlapping copies are calledepimers.
From a physical point of view, the stereoisomers of a molecule all have the same properties, for example color, melting point, and so on. And chemically all react in the same way with non-chiral molecules.
What changes, however, is the way they react with other chiral molecules. Since organic, chiral molecules are in abundance, the fact that a compound is an epimere rather than another makes a significant difference. For example, among sugars, the biologically important ones are predominantly those of the D series characterized by a specific orientation of one of the H-C-OH groups.
The scholar who has made a fundamental contribution to this field of chemistry is Emil Fischer.
In his stereoisomer studies, Fischer used what was later called Fischer Projection.
This is a set of conventions to represent in the plane the positions of atoms in space.
In the following picture you can see a simplified molecule showing a chiral center, through which the projection plane passes, and 4 functional groups A, B, X, and Y which are linked to the chiral center.
In the projection process the four groups come to form a cross in which the segments A and B, besides being to the right and left of the center, are also OVER the projection plane, while the other two are under.
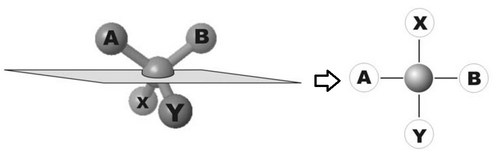
In this representation, swapping AB with XY means tripling the projection plane, that is to convert an enantiomer into the other.
Fischer applied this technique to the representation of glyceraldehyde leading to the definition of D-glyceraldehyde and L-glyceraldehyde.
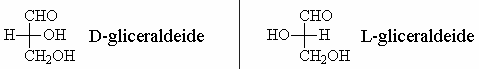
As we have said, biologically relevant sugars are those of the “D” series, but also the amino acids have stereoisomeric characteristics and also in this case, in the living organisms, only one family, called “L”, is actually used in cells.
 -0
-0  )
)