Glyceraldehyde
Glyceraldehyde is a carbohydrate aldo-triose, consisting of three carbon atoms and containing an aldehyde group (CHO).
The name derives from the fact that the molecule of glyceraldehyde is similar to that of glycerol whose formula is
CH2OH-CHOH-CH2OH (Glycerol)
Where one of the two CH2OH groups has been replaced by a CHO group by obtaining
CH2OH-CHOH-CHO (Glyceraldehyde)
An important feature of glyceraldehyde is its chirality, that is, its molecule can exist in two specular spatial configurations.
Spatial asymmetry turns into the property of polarizing the light that crosses it in a sense rather than in the other, ie right or left.
For this reason, the two configurations are called D and L and are conventionally represented as shown.
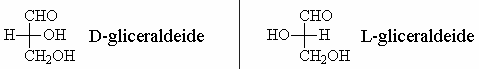
D-glyceraldehyde is used as a model for the nomenclature of carbohydrates.
The carbohydrates of the D series are those whose arrangement of the constituents around the penultimate carbon atom is similar to that of D-glyceride. Those with specular configuration in the last chiral carbon are carbohydrates of the L series.
This classification and nomenclature is due to the work of the German chemist Nobel laureate for Chemistry in 1902 Hermann Emil Fischer for his chemistry studies on sugars.
Fischer observed the effects of the chirality of the sugar molecules on the light polarization, so it was clear that the spatial arrangement of the atoms in the various molecules was different but, with the instruments at his disposal, he was not able to distinguish between enantiomeric sugars.
He then introduced the convention that the glyceraldehyde (+) and (-) stereoisomers were designated respectively as:
- D-glyceraldehyde -> The stereoisomer with the hydroxyl group on the C2 to the right
- L-glyceraldehyde -> The stereoisomer with the hydroxyl group on the C2 to the left
Knowing that there was only 50% of the chances that this assignment was correct.
Fischer then assumed that the configuration of these molecules was the one shown in the figure.
Later, in 1954, with the advent of X-ray diffraction, Bijvoet determined the structure of D-tartaric acid and thus determined the absolute configuration and found that the Fischer assumption (hydroxyl group on the right in the (+)-glyceraldehyde) was correct.
& Nbsp;
 -0
-0  )
)