Materials: PP (polypropylene)
The polypropylene suitable for commercial uses was born in Italy, thanks to the contribution of prof. Giulio Natta, who was awarded with the Nobel Prize in 1963 for his discovery of stereospecific catalysts for the polymerization.
The use of these catalysts allows to control the spatial arrangement of the monomers during the polymerization reaction and, in this case, allows the creation of isotactic polypropylene.

As you can see in the image, the monomer of propylene has a methyl group [-CH3] which can be in different relative position with respect to the methyl groups of the adjacent monomers.
We have essentially two cases:
atactic polypropylene, wherein the CH3 groups are arranged randomly along the chain

and isotactic polypropylene, wherein the CH3 groups are neatly arranged on the same side
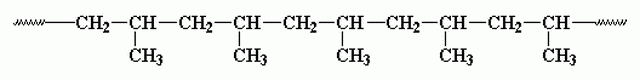
Of the two versions, the isotactic polypropylene is the one that has the most favorable mechanical properties and it is the Natta stereospecific catalyst that allows the polymerization to occurs selectively in this form.
The polypropylene thus formed was marketed by Montecatini with the name of MOPLEN and is still one of the most widely used polymers for various household goods.
In fact, the polypropylene has a resistance to a temperature above the polyethylene, reaching 160°C (320°F). For this reason it is used for making dishes and containers for foods that can be washed in the dishwasher. Being easy to wash and not absorbing water, it is used as a coating for outdoor and garden furniture. Furthermore, the PP can be processed into fiber, thus is used to make carpets and moquettes.
Below some images of objects made of polypropylene.




 -0
-0  )
)
Leave a Reply